valence electrons of s|Iba pa : iloilo Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s 1 s sublevel are .
Envelope design. Harvest and fundraising. Fundraising Envelope Design. Harvest Envelope Design. Church Envelope Design. Fundraising Flyer Design. Envelope Design Template. Corel Draw Tutorial. Rollup Design. Funeral Posters. Christian Graphic Design. NKD Graphix. 1k followers.It is an offline game which you can play tongits on anytime and anywhere. The objective of the game is to empty your hand of all cards or minimize the count and the scores of unmatched cards that are still on the player's .
valence electrons of s,Mar 23, 2023 Find the valences of the elements in a table that shows the maximum and . Valence electrons are the s and p electrons in the outermost shell. The electrons present in the inner shell are core .

In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a chemical bond if the outermost shell is not closed. In a single covalent bond, a shared pair forms with both atoms in the bond each contributing one valence electron. The presence of valence electrons can determine the element's chemical prope. There are two ways to find the number of valence electrons in Sulfur (S). The first is to use the Periodic Table to figure out how many electrons Sulfur has in its valence shell. To do.
Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s 1 s sublevel are .An atom's valence electrons are the electrons in its outermost shell. In the chlorine model below, the valence electrons are shown in red . A Bohr model of a chlorine atom . Learn how to identify the valence electrons of atoms, which are the electrons in the outermost shell that participate in chemical reactions. Watch a video and read the comments from other learners and experts on this topic. Define valence electrons. Define the octet rule. Determine the most likely ion of an element. A chemical reaction involves either electron removal, electron . Introduction to Chemistry. 11: Chemical Bonding. 11.1: Valence Electrons and the Periodic Table. Expand/collapse global location. 11.1: Valence Electrons and the Periodic Table. Page ID. ⚙️ . sulfur. helium. potassium. aluminum. Solution. Sulfur (S) is located in Group VIA (Group 16), so it has 6 valence electrons. Helium (He) is located in Group VIIIA (Group 18). However, one atom only has two electrons, so it could never have more than 2 valence electrons. As noted above, helium is the only exception for the main group . As a gas or vapor, the halogens all had a pungent odor. After the development of quantum mechanics, it was shown that the halogens all had seven valence electrons, supporting their original placement into .Iba pa There are 2 electrons in the 2s subshell and 2 electrons in the 2 p subshell, giving carbon a total of four valence electrons. Bromine’s ground state electron configuration is 1s 2 2s 2 p 6 3s 2 p 6 d 10 4s 2 .Characteristics of Valence Electron. Electrons are involved in the chemical bonding and reactions of the atom. It is said to occupy orbitals in an atom. The number of valence electrons of an atom can be obtained from the periodic table because it is equal to the group number of the atom. Atoms are most stable if they have a filled valence shell .
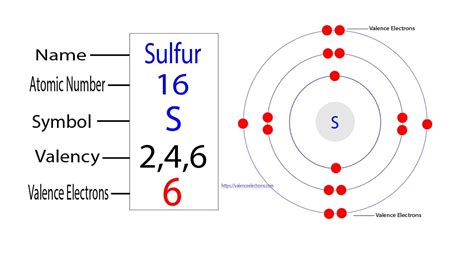
Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s 1 s sublevel are called inner-shell electrons and are not involved directly in the element's reactivity, or in the formation of compounds. Lithium has a single electron in the second . Step 4: Find Valence Electrons. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of six electrons present in the valence shell of sulfur (3s²3p⁴). Thus, sulfur has six valence electrons. Also Read: Valence Electrons & Valency of Oxygen (O).
Valence Electrons. As was mentioned in a previous section of this chapter, electrons are highly important, because a specific subset of electrons, called valence electrons, are solely-responsible for determining how elements bond with one another.The number of valence electrons that are present in an atom can be determined from that atom's . Wade’s rules. valence electron, any of the fundamental negatively charged particles in the outermost region of atoms that enters into the formation of chemical bonds. Whatever the type of chemical bond (ionic, covalent, metallic) between atoms, changes in the atomic structure are restricted to the outermost, or valence, electrons.
Generally, valence electrons can participate in the formation of chemical bonding, but core electrons cannot. While core electrons are not involved in bonding, they influence the chemical reactivity of an atom. The electron configuration of a oxygen atom is. O: 1s22s22p4 (1.9B.1) (1.9B.1) O: 1 s 2 2 s 2 2 p 4. which may be shorted.

The stronger pull (higher effective nuclear charge) experienced by electrons on the right side of the periodic table draws them closer to the nucleus, making the covalent radii smaller. Figure 8.4.2 8.4. 2: Within each period, the trend in atomic radius decreases as Z increases; for example, from K to Kr.valence electrons of s Iba pa 6 Sulfur has six valence electrons. Valence electrons are the outermost electrons which, therefore, are located on the highest energy levels. Consequently, these are the electrons available for chemical bonding. To determine the valence number, look at electron configurations which denote the number of electrons in the different energy .
Valence Electrons. The electrons in the outermost shell are the valence electrons the electrons on an atom that can be gained or lost in a chemical reaction. Since filled d or f subshells are seldom disturbed in a .The number of electrons in the outermost shell of a particular atom determines its reactivity, or tendency to form chemical bonds with other atoms. This outermost shell is known as the valence shell, and the electrons found in it are called valence electrons. In general, atoms are most stable, least reactive, when their outermost electron shell .
2. Find the electron configuration for the element you are examining. Once you know an element's electron configuration, finding its number of valence electrons is quite simple (except, of course, for the transition metals.) If you're given the configuration from the get-go, you can skip to the next step.The valence electrons are the electrons that determine the most typical bonding patterns for an element. These electrons are found in the s and p orbitals of the highest energy level for the element. Sodium 1s22s22p63s1. Sodium has 1 valence electron from the 3s orbital. Phosphorus 1s22s22p63s23p3. Phosphorus has 5 valence electrons 2 from the .
Valence electrons are the electrons present in the outermost shell of an atom. You can easily determine the number of valence electrons an atom can have by looking at its Group in the periodic table. For example, atoms in Groups 1 and 2 have 1 and 2 valence electrons, respectively. Atoms in Groups 13 and 18 have 3 and 8 valence electrons .valence electrons of sValence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s 1 s sublevel are called inner-shell electrons and are not involved directly in the element's reactivity, or in the formation of compounds. Lithium has a single electron in the second principal . And so for this video, we're only talking about the valence electrons for elements in the main groups. When we talk about the main groups, you're using the one through eight system .
valence electrons of s|Iba pa
PH0 · which element has 3 valence electrons
PH1 · valence electrons chart
PH2 · valence electrons calculator
PH3 · valence electron configuration calculator
PH4 · periodic table showing valence electrons
PH5 · list of valence electrons for each element
PH6 · how many valence electrons does al have
PH7 · how do you find valence electrons
PH8 · Iba pa